This is an old revision of the document!
Heat: Energy Transfer due to a Temperature Difference
Earlier, you read about work, which is the mechanical energy transfer of energy into or out of a system. This energy transfer is due to physical displacements of the system over macroscopic distances by the external forces acting on the system. When you place a hot object near a cold one, you observe an energy transfer from the hot object to the cold object, but this is not due to macroscopic work (i.e., pushes and pulls over observable distances). It is instead due to the microscopic work done by the atoms in contact at the boundary. In time, this microscopic work propagates through the whole cold object. In these notes, you will read about that process.
Two blocks in contact
Two blocks are placed in contact (figure to right). One block is hot (red) and the other is cold (blue). Your experience tells you that in time, the hot block will become cooler and the cold block will become warmer. Eventually, the two blocks will achieve the same (intermediate) temperature.
How does this happen?
The average kinetic energy of atoms in the high temperature block is higher than the low temperature block. Some of this energy is due to the random motions of the atoms, which we refer to as thermal energy. So on average, the atoms in the high temperature block are jiggling more, and thus have more thermal energy per atom.
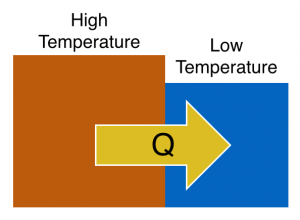
At the interface, faster moving atoms in the high temperature block collide with slower moving atoms in the low temperature block transferring energy across the boundary. This atoms collide with their neighbors within their own block and the energy is propagated across the rest of the block. You can think about this as work done by motion of atoms, but it's not macroscopic work in the sense that you read about earlier. It is instead the microscopic work done by atomic collisions, which we refer to as $Q$ – the energy transfer due to a temperature difference.
Because each atom has a different kinetic energy, it is possible that a fast moving atom from the low temperature block will transfer kinetic energy to a slow moving atom in the high temperature block. However, this is less likely than the reverse because they are more atoms on average with high kinetic energies in the high temperature block.
The net result is a energy transfer across the boundary of the two blocks from the hot block to the cold block. This is also referred to as the heat exchanged, but be careful using the word heat as it has colloquial definitions that are not scientific. For example, an object doe not have heat, it has thermal energy.